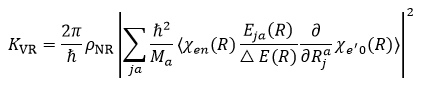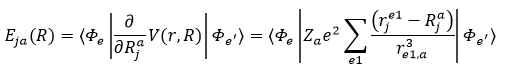Current Issue
Enhancing Upconversion Luminescence in Lanthanide Nanocrystals by Using Organic Ligands to Inhibit Vibrational Relaxation
Ni Zhang1,2,3, Guoqin Ma2, Jin Xu1,2,3,*
1College of Physics and Energy, Fujian Normal University, Fuzhou, Fujian 350007, China
2State Key Laboratory of Structural Chemistry, Fujian Key Laboratory of Nanomaterials, and Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China
3Fujian College, University of Chinese Academy of Sciences, Fuzhou, Fujian 350116, China
*Corresponding author: Dr. Jin Xu, Associate Professor, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou, Fujian 350002, China, Phone: 15880031586, E-mail: [email protected]
Received Date: April 26, 2025
Publication Date: May 08, 2025
Citation: Zhang N, et al. (2025). Enhancing Upconversion Luminescence in Lanthanide Nanocrystals by Using Organic Ligands to Inhibit Vibrational Relaxation. Material Science. 7(1):38.
Copyright: Zhang N, et al. © (2025).
ABSTRACT
Lanthanide nanocrystals are capable of converting low-energy photons into high-energy photons via a multi-photon mechanism, which renders them highly promising for applications in bioimaging/detection and photodynamic therapy. However, the practical bioapplication of lanthanide upconversion nanocrystals is hindered by their low upconversion luminescence efficiency and susceptibility to temperature-induced luminescence quenching. Recent studies have demonstrated that the upconversion luminescence intensity can be significantly enhanced through the coordination of small organic molecules, such as picolinic acid (2PA), on the surface of these nanocrystals. Despite this advancement, the underlying physical mechanism of the enhancement induced by organic ligands remains elusive. In this paper, we state our viewpoints on the phenomenon of ligand-induced upconversion enhancement.
Keywords: Organic Ligand, Surface Coordination, Lanthanide Nanocrystals, Upconversion Luminescence
INTRODUCTION
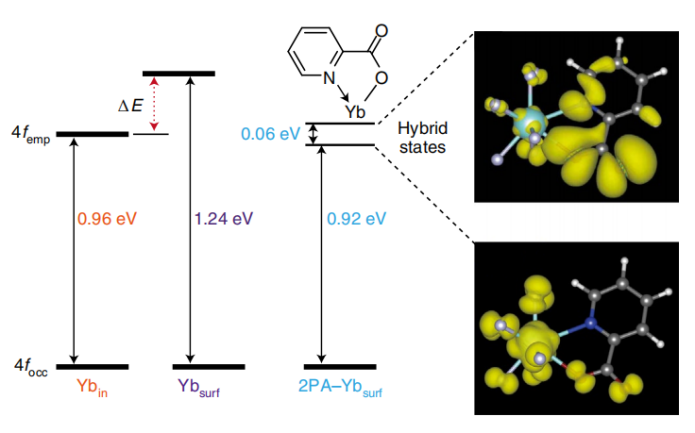
In 2021, Liu et al. [1]. developed a novel surface engineering strategy to enormously amplify the upconversion luminescence of lanthanide nanocrystals (NaGdF4:Yb3+/Tm3+) through the chelating effect of organic small-molecule ligand (e.g., picolinic acid). The observed enhancement was initially rationalized through molecular orbital hybridization theory. According to this hypothesis, the orbital hybridization between coordination-unsaturated surface lanthanide ion and organic ligand eliminates the energy-level mismatch between the 2F5/2 states of surface and inner Yb3+ ions (4f13 configuration), thereby facilitating the resonance energy transfer between surface and inner Yb3+ ions. Consequently, the multi-photon upconversion process of Yb3+ → Tm3+ is substantially enhanced (Figure 1). However, this explanation appears inconsistent with the observed decrease in the transition probability of 2F5/2 level of Yb3+ ions after ligand coupling on the nanocrystal surface (corresponding to the increase in the fluorescence lifetime of Yb3+ ions at 980 nm).
From the crystal structure perspective, it is well accepted that the breaking translational symmetry in lanthanide fluoride nanocrystal is bound to result in the unsaturated coordination of surface lanthanide ions. Accordingly, upon the chelating of picolinic acid ligands, they will behave as pseudoatoms to support the crystal field for surface lanthanide ions. Thus, it is conceivable that the coordination ligands may affect the surface electronic structures in lanthanide nanocrystals through the perturbation of electrostatic crystal field of ligand to lanthanide inner 4f electronic configuration, or the orbital hybridization of ligand with lanthanide ion. However, in the cases absent of strong spin-orbit coupling caused by the heavy atoms of coordination anions (such as I-), the energy levels for the inner 4f electrons of surface Yb3+ ions are barely affected by the surrounding crystal field due to the shielding effect of outer 5s and 5p electrons. Indeed, no observable difference can be found in the emission peak positions corresponding to Yb3+ 2F5/2 → 2F7/2 intra-4f transitions between the nanocrystals before and after surface chelating of picolinic acid ligands. Therefore, we do not expect the orbital hybridization between coordination-unsaturated surface lanthanide ion and organic ligand accounts for the anomalous phenomenon of ligand-induced upconversion enhancement.
Figure 1. Left: simulated single-particle 4f energy levels of ytterbium atoms with nine-, six- and eightfold coordination configurations, respectively. 4femp and 4focc represent the lowest empty and highest occupied 4f orbitals of the ytterbium atom, respectively; ΔE is the energy difference between the 4femp-4focc gaps of the surface and inner ytterbium atoms. Right: spatial distribution of the partial charge densities of coupling states formed by coordination between Yb3+ ion and picolinic acid ligand. Cyan, purple, grey, white, red and blue balls denote Yb, F, C, H, O and N atoms, respectively [1].
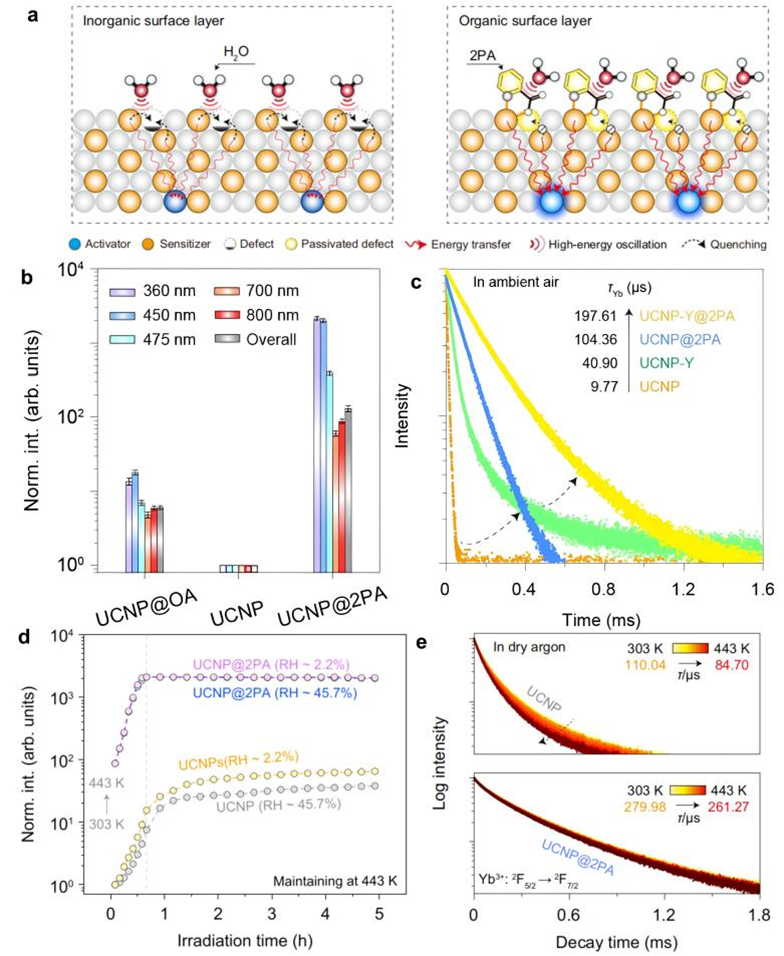
Recently, Wang et al. [2]. performed a combined experimental investigation and theoretical calculation to confirm that the picolinic acid bidentate ligand coupled to the surface Yb3+ ion of lanthanide nanocrystal can effectively shield the nanocrystal from high-frequency vibration of water molecule. This prevents the vibration-induced non-radiative relaxation process of the 2F5/2 excited state of Yb3+ ions, resulting in the enhancement of upconversion luminescence (Figure 2a). Specifically, upon 980-nm laser excitation, the Tm3+ ion ultraviolet upconversion luminescence (corresponding to the 1D2 → 3H6 transition) was enhanced by approximately 2100 times in the NaGdF4:Yb3+/Tm3+ upconversion nanocrystals (dispersed in ethanol) capped with picolinic acid ligand as compared to nanocrystals without surface ligand (Figure 2b). It is reasonable to infer that the inhibition of vibrational relaxation of Yb3+ 2F5/2 state leads to a decrease in its transition probability, which complies with the experimental observation of an increase in its fluorescence lifetime (Figure 2c). In fact, the inhibition of vibrational relaxation of lanthanide ions by surface ligands can be well explained theoretically. The vibrational relaxation rate KVR of the 2F5/2 excited state of Yb3+ ions are assumed to be [3]:
Where j refers to x, y, or z, ρNR is a density of final state vibrational levels, Za is the nuclear charge of atom a, Ma is the mass of atom a, V(r, R) describes the interaction between electrons and nuclei, χe’0 (R) and χen(R) are vibrational eigenfunctions of initial and final states, Δ E(R) is the energy difference between the initial and the final states, Φe’ and Φe are the electronic wave functions of initial and the final states. Apparently, the KVR decreases inversely with the sixth power of distance between Yb3+ ion and water molecule, and scales positively with the gradient of vibrational eigenfunction χe’0 (R) (i.e.,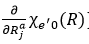 ). It is noteworthy that the gradient of χe’0 (R) denotes the reciprocal of rigidity of picolinic acid that chelate with surface Yb3+ ion. Therefore, upon the surface chelating of picolinic acid ligand, the steric hindrance of ligand enlarges the effective distance between Yb3+ ion and water molecule, and meanwhile the rigid five-membered ring structure formed by the coordination of lanthanide ion with picolinic acid suppresses the phonon interactions between picolinic acid and water molecule, both of which results in the inhibition of vibrational relaxation of Yb3+ ions induced by the high-frequency phonon modes of water molecule.
). It is noteworthy that the gradient of χe’0 (R) denotes the reciprocal of rigidity of picolinic acid that chelate with surface Yb3+ ion. Therefore, upon the surface chelating of picolinic acid ligand, the steric hindrance of ligand enlarges the effective distance between Yb3+ ion and water molecule, and meanwhile the rigid five-membered ring structure formed by the coordination of lanthanide ion with picolinic acid suppresses the phonon interactions between picolinic acid and water molecule, both of which results in the inhibition of vibrational relaxation of Yb3+ ions induced by the high-frequency phonon modes of water molecule.
Additionally, they discovered that the lanthanide upconversion nanocrystals capped with picolinic acid ligands exhibited significant upconversion enhancement even at high temperatures (Figure 2d). Notably, during the continuous heating from 303 to 443 K, the humidity impacted the upconversion enhancement because the water coordination under a higher humidity condition reduced the vibrational relaxation inhibition capability of picolinic acid ligand, and intriguingly, the nanocrystals capped with picolinic acid ligands rapidly reached peak luminescence intensity upon laser excitation and maintained stability for the subsequent 5 hours, demonstrating excellent thermal stability (Figures 2d-e). This is because the five-membered ring chelate structure formed by the coordination of surface lanthanide ion with picolinic acid inhibits the non-radiative dissipation process of excitation energy induced by the thermal field. Nevertheless, if the temperature further exceeds 443 K, the risk of de-chelating and even carbonization of picolinic acid ligand will increase sharply.
Despite these advances, two fundamental questions persist: first, the precise phononic structure of surface lanthanide-picolinic acid chelates and their interaction mechanisms with solvent vibrational modes remain unresolved; second, the design principles for next-generation ligands (such as ligands with larger coordination number and higher chelation strength, more rigidity, and more hydrophobic groups) surpassing picolinic acid's performance in aqueous environments require systematic exploration. Addressing these challenges is paramount for overcoming the intrinsic quantum yield limitations of rare-earth upconversion systems, particularly for advancing their biomedical applications in high-sensitivity bioimaging [4-7], real-time biosensing [8-9], and precise photodynamic therapy.
Figure 2. (a) Schematic illustration of upconversion enhancement by an organic surface layer. Left: small nanocrystals typically feature low efficiency due to severe surface quenching through defects (e.g., vacancies) and high-energy oscillators (i.e., -OH). Right: after picolinic acid (2PA) coordination, the organic surface layer can passivate defects and isolates high-energy oscillations, giving rise to high brightness over a wide temperature range [2]. (b) Peak intensities of Tm3+ emissions at different wavelengths and overall integrated intensity for ligand-free, oleic acid (OA)-coated and 2PA-capped NaGdF4:Yb3+/Tm3+ (49/1%) colloidal nanocrystals. (c) Luminescence decay curves of Yb3+: 2F5/2 → 2F7/2 transition from untreated and solution-annealed NaGdF4:Yb3+/Tm3+ (49/1%) nanocrystals after 2PA modification measured in ambient air. (d) Normalized integral intensity of overall emissions (300-900 nm) at 443 K against the 980 nm laser irradiation time in ligand-free and 2PA-capped NaGdF4:Yb3+/Tm3+ (49/1%) nanocrystals under different relative humidity conditions. Note that the heating process from 303 to 443 K took around 40 min. (e) Luminescence decay curves of Yb3+: 2F5/2 → 2F7/2 transition in ligand-free (top panel) and 2PA-capped nanocrystals (bottom panel) as a function of temperature measured in dry argon [2]. Among these, UCNP denotes NaGdF4:Yb3+/Tm3+ (49/1%) nanocrystals, UCNP@OA, UCNP@2PA and UCNP-Y represent OA-coated, 2PA-capped and solution-annealed NaGdF4:Yb3+/Tm3+ (49/1%) colloidal nanocrystals, respectively.
AUTHOR CONTRIBUTIONS
Ni Zhang: Writing-original draft, Methodology, Validation, Investigation, Formal analysis. Guoqin Ma: Methodology, Formal analysis. Jin Xu: Funding acquisition, Writing-review & editing, Supervision, Conceptualization.
FUNDING SOURCES
Project supported by the National Natural Science Foundation of China (Nos. 12374389, 12074380) and Youth Innovation Promotion Association of Chinese Academy of Sciences (No. 2022306).
NOTES
Any additional relevant notes should be placed here.
REFERENCES
- Xu H, Han S, Deng R, Su Q, Wei Y, Tang Y, et al. (2021). Anomalous upconversion amplification induced by surface reconstruction in lanthanide sublattices. Nat. Photon. 15:732-737.
- Suo H, Zhao P, Zhang X, Guo Y, Guo D, Chang J, et al. (2025). Bright upconversion over extended temperatures enabled by an organic surface layer. Nat. Commun. 16:3249.
- van Dijk JMF, Schuurmans MFH. (1983). On the nonradiative and radiative decay rates and a modified exponential energy gap law for 4f-4f transitions in rare-earth ions. J Chem Phys. 78:5317-5323.
- Yang S, Qi B, Sun M, Dai W, Miao Z, Zheng W, et al. (2024). Broadband Near-Infrared Light Excitation Generates Long-Lived Near-Infrared Luminescence in Gallates. ACS Nano. 18(33):22465-22473.
- Li D, Jia M, Jia T, Chen G. (2024). Ultrasensitive NIR-II Ratiometric Nanothermometers for 3D In Vivo Thermal Imaging. Adv Mater. 36(11):e2309452.
- Qin X, Carneiro Neto AN, Longo RL, Wu Y, Malta OL, Liu X. (2021). Surface Plasmon-Photon Coupling in Lanthanide-Doped Nanoparticles. J Phys Chem Lett. 12(5):1520-1541.
- Liu C, Zhang X, Chen X, Liang L. (2024). Emerging Advances in Lanthanide Photon Avalanche Nanophotonics. Nano Lett. 24(49):15489-15500.
- Ansari AA, Parchur AK, Li Y, Jia T, Lv R, Wang Y, et al. (2024). Cytotoxicity and genotoxicity evaluation of chemically synthesized and functionalized upconversion nanoparticles. Coord Chem Rev. 504:215672.
- Kumar B, Malhotra K, Fuku R, Van Houten J, Qu G, Piunno PAE, et al. (2021). Recent trends in the developments of analytical probes based on lanthanide-doped upconversion nanoparticles. TrAC Trends Anal Chem. 139:116256.
 Abstract
Abstract  PDF
PDF