Past Issues
The Characterization and Mercury Oxidation Performance of Bromine-Doped Vanadia/Titania Oxide Catalyst
Yuntao Li1*, Gong Chen1, Xuanxiong Kang1, Linzhi Zhai2*, Shuling Dong3, Mengjun Huang1, Hongpan Liu1
1Chongqing Key Laboratory of Environmental Materials and Remediation Technologies, College of Chemistry & Environmental Engineering (Chongqing University of Arts and Sciences), Chongqing, PR China 2School of Environmental and Chemical Engineering, Jiangsu University of Science and Technology, Zhenjiang, PR China 3School of Chemistry, Biology and Materials Engineering, Suzhou University of Science and Technology, Suzhou, PR China
∗Corresponding authors: Yuntao Li, Chongqing Key Laboratory of Environmental Materials and Remediation Technologies, College of Chemistry & Environmental Engineering (Chongqing University of Arts and Sciences), Chongqing 402160, PR China. Linzhi Zhai, School of Environmental and Chemical Engineering, Jiangsu University of Science and Technology, Zhenjiang 212000, PR China
Received: November 10, 2019 Published: November 26, 2019
ABSTRACT
Mercury pollution produced by coal combustion has been identified as a severe hazardous pollutant to human health and the environment. For the coal-fired power plants, one of the most cost-effective methods to control mercury is to use SCR catalysts, which can achieve both denitration and oxidation effects, simultaneously. In the present study, a new SCR catalyst, bromine-doped vanadia/titania oxide, has been developed, and its structure and mercury oxidation characteristics were systematically studied. Results demonstrated that, in compare to the control sample, bromine-doped vanadia/titania oxide has obviously higher amount of V4+ and Ti3+, thus resulting in an improved redox property. Activity tests showed that the catalytic capacity was highly dependent on the doping amount of bromine with a specific feature from ‘low to high to low’, The highest value for mercury oxidation of the product is [Br]/[Ti]=1.2×10-2.
Keywords: Minamata Convention; Mercury control; SCR denitration catalyst; Bromine doping
INTRODUCTION
Mercury and its compounds have considerable harm to the human digestive system, central nervous system and kidneys, and are one of the persistent environmental pollutants that are currently receiving sustained attention. One of the main sources of mercury in the atmosphere is coal-fired emissions. On January 19, 2013, the United Nations Environment Program adopted the Minamata Convention, an international convention for the control and reduction of mercury emissions worldwide. The Convention requires the control of mercury emissions from various large coal-fired power station boilers and industrial boilers. Therefore, mercury emission control of coal combustion is one of the hot spots of environmental protection research.
Mercury control methods in coal-fired power plants can be divided into mercury removal before combustion (pre-stage technology), mercury removal during combustion, and mercury removal after combustion (back-end technology). Among them, the widely used technologies include bromine-treated coal before combustion and injecting brominated activated carbon into the flue gas after combustion, these two technologies have been industrially applied abroad, but the investment and operating costs are high [1]. On the other hand, coal-fired power plants in China are basically equipped with selective catalytic reduction (SCR) denitration equipments, electrostatic precipitators and wet desulfurization facilities, and a large number of field test results [2] show that the combined use of SCR, electrostatic precipitator and wet desulfurization facilities could effectively reduce gaseous mercury emissions. The author has also tested three power plants in China and found that the mercury removal efficiency of the electrostatic precipitator system is 7.85%~44.63%, and the mercury removal efficiency of the wet desulfurization system is 43.63%~75.35% (the mercury was basically all present in the gypsum). Less than 20% of the mercury was emitted and was mainly elemental mercury. These test results fully demonstrate that the use of installed SCR, electrostatic precipitator and wet desulfurization facilities to achieve synergistic mercury removal is an effective and low-cost mercury removal technology.
The key to achieving synergistic mercury removal by SCR, electrostatic precipitator and wet desulfurization facilities is to develop a SCR catalyst with high elemental mercury oxidation rate. Existing commercial SCR catalysts have been proven over the long term and improvements based on existing commercial SCR catalysts will significantly reduce the difficulty of mercury control engineering applications. When testing the commercial V2O5-WO3/TiO2 honeycomb SCR catalyst, the authors found that although the catalyst has a good deNOx effect, the oxidation rate of mercury was less than 10% (test conditions are based on the VGB test standard). The results of Schwämmle et al. [3] has shown that increasing the wall thickness of the honeycomb catalyst could increase the mercury oxidation capacity of commercial SCR catalysts. But this is equivalent to increasing the amount of catalyst, which leads to an increase in SO2 conversion. So, how should the mercury oxidation performance of existing commercial SCR catalysts be improved?
The mechanism of oxidation of elemental mercury on the surface of SCR catalysts yielded different results under different catalysts and different test conditions, Eley–Rideal mechanism [4], Langmuir–Hinshelwood mechanism [5-8] and Mars–Maessen mechanism [9,10 ] were possible. The oxidation of elemental mercury on the surface of the SCR catalyst is a heterogeneous catalytic reaction process. Regardless of the reaction mechanism, the adsorption of the reactants and the redox capability of the catalyst are core. Theoretical results showed that on the surface of oxygen defects, the adsorption energy of Hg0 was much higher than that on the clean surface, which was a strong chemical adsorption; while the doping caused the incompletely coordinated O atoms to have strong adsorption to Hg. The presence of surface active oxygen species plays an important role in the adsorption of mercury [10, 11]. The mercury oxidation process promoted the conversion of V5+ species into V4+ species and consumed lattice oxygen on the catalyst surface [12]. The transfer efficiency of electrons between V5+, V4+ and other catalyst components such as vanadium and titanium has an important influence on the mercury oxidation reaction. Therefore, we can further study the SCR catalysts with high elemental mercury oxidation rate from the promotion of the adsorption of elemental mercury by SCR catalysts and the redox capacity of SCR catalysts.
At present, the main idea in the study of SCR catalysts for elemental mercury oxidation is to add different transition metal oxides or combinations thereof [13], such as Co-Mn[10], CeO2 [14,15], Mo-Ru [16], RuO2 modified Ce-Zr complex [17], Au/TiO2 [18], CuCl2/γ-Al2O3 [19], Graphene enhanced Mn-Ce binary metal oxides [20], Fe2O3 [21,22], CuCl2-CoOx/Ti-CeOx [23], Co–MF [24], manganese oxide octahedral molecular sieve [25], Mn-Fe co-modified ZSM-5 [26], etc. These studies are important in how to promote the adsorption of the catalyst on elemental mercury and improve the redox capacity of the catalyst. However, is there any other way to strengthen the mercury oxidation capacity of the SCR catalyst? Existing mature mercury removal technology, coal treated by bromine can promote the oxidation of elemental mercury in the combustion process, brominated activated carbon can enhance the adsorption and oxidation of elemental mercury and brominated activated carbon is better than chlorinated activated carbon, HBr in the flue gas is better than HCl for mercury oxidation [27], which showed the importance of non-metallic bromine in mercury control. In view of this, this paper studies the effect of non-metallic bromine doping on the structure and performances of vanadia/titania catalyst from a non-metallic perspective, exploring new ideas for elemental mercury oxidation by SCR catalysts.
EXPERIMENTAL
Preparation of Br-doped V/TiO2
The bromine-doped carrier titanium oxide was first prepared by the sol-gel method, and then vanadium oxide was impregnated. Typical experiment, as follows: First, 21.7830 g (0.064 mol) of butyl titanate (C16H36O4Ti) was added into 0.128 mol of acetylacetone (acacH, C6H8O2) solution, keeping the molar ration of [acacH]/[Ti]=2. Then, 50 mL ammonium bromide (NH4Br)/ethanol mixed solution was poured to above C16H36O4Ti/ C6H8O2 solution, stirring for 2 hours to form a sol. Thereafter, the sol product was heated at 333 K, followed by evaporating for 6 h at 393 K. The resultant product was calcined at 773K for 3 h, the Br-doped TiO2 powder was obtained. Finally, the Br-doped TiO2 powder (particle size was 0.70-0.90mm) was immersed in NH4VO3 aqueous solution, refluxing for 4 h at 333 K, and dried for 6 h at 393 K, sequently. The powder was calcined at 623 K for 4 h to obtain a Br-doped V/TiO2 catalyst. The catalyst sample is designated as VTiBr x, (where x represents 100 × [Br]/[Ti], [Br]/[Ti] = 0 ~ 2 × 10-2).
Characterrization of Br-doped V/TiO2
The X-ray photoelectron spectroscopy (XPS) was performed by the ESCALAB 250Xi device of ThermoFisher Scientific. The X-ray diffraction spectrum (XRD) was tested by the D8 X-ray diffractometer of Bruker. The specific surface area and pore structure were determined by the ASAP2460 of Micromeritics. The electronic paramagnetic resonance (EPR) spectrum of the sample was detected by the A300 equipment of Bruker. The elemental composition of the sample is quantitatively analyzed by Agilent's Agilent 725 series ICP-OES equipment. The Photoluminescence spectrum (PL) was tested by the FLs980 full-function steady-state/transient fluorescence spectrometer from Edinburgh Instruments.
Mercury oxidation performance test
The mercury oxidation performance test was carried out in a fixed-bed reactor (inner diameter 6.8 mm). The experimental scheme was shown in (Figure 1). 0.3 g catalyst was filled, then the simulated flue gas (3×10-5 μg/mL Hg + 5% O2, and the rest was N2 flow) was passed at 150 mL/min The Hg was placed into a quartz U-tube with a mercury permeation tube (VICI Metronics, USA) embedded in an isothermal water bath, then mercury vapor was provided into the gas stream through N2 current.
Before the mercury oxidation performance test, the simulated flue gas was introduced into the fixed-bed reactor for 48 h to ensure the Hg-adsorption saturation by the Br-doped V/TiO2. The set temperature was stable for more than 1h, and the concentration of Hg was analyzed by the coal-fired flue gas mercury analyzer QM201H.
Hg conversion (X) is defined as:
X = (Hgin – Hgout) / Hgin (1)
The reaction kinetic order for Hg0 in the SCR catalyst oxidation, elemental mercury reaction was one [28], and the reaction kinetic order of O2 was zero. Thus, the reaction rate of oxidizing Hg0 is:
r = kCHg0 (2)
In order to compare the activity of different vanadia-based catalysts, the rate constant km was used as an evaluation index:
Where 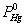 was the partial pressure of Hg0;
was the partial pressure of Hg0; 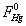 was the molar flow of Hg0 at the inlet of the reactor; wm is the mass of vanadium [29].
was the molar flow of Hg0 at the inlet of the reactor; wm is the mass of vanadium [29].
Figure 1: Mercury oxidation performance test experimental procedure.
RESULTS AND DISCUSSION
Pore structure and composition analysis
The vanadia content was detected by ICP analysis (see Table 1). The results indicated that the active vanadia content of the Br-doped V/TiO2 was 0.60~0.75 1%, which was lower than some conventional V-based SCR catalysts. The surface vanadium density (ns) was small, and the active component was in an effective dispersion state.
It was found that the vanadia content of commercial SCR catalysts used in China was generally 0.3~1.5% to ensure the high deNOx efficiency, small SO2 oxidation and ammonia slip. Therefore, the preparation of SCR catalysts with lower vanadium content and high mercury oxidation efficiency still has large challenges.
Table 1: Vanadia content, BET specific area, ns and the kinetic constants of the catalysts.
|
V2O5 (%wt) |
SBET (m2 g-1) |
ns (VOx nm-2)a |
km (593K) (mol Hg(g V)−1 Pa−1 s−1) |
km (623K) (mol Hg(g V)−1 Pa−1 s−1) |
|
|
VTiBr 0 |
0.62 |
20.41 |
2.01 |
2.52×10−6 |
1.71×10−6 |
|
VTiBr 0.4 |
0.60 |
24.99 |
1.59 |
3.93×10−6 |
3.11×10−6 |
|
VTiBr 0.8 |
0.75 |
22.12 |
2.24 |
4.67×10−6 |
3.38×10−6 |
|
VTiBr 1.2 |
0.69 |
21.59 |
2.11 |
9.91×10−6 |
6.76×10−6 |
|
VTiBr 1.6 |
0.63 |
25.99 |
1.60 |
7.61×10−6 |
5.23×10−6 |
|
VTiBr 2 |
0.69 |
27.44 |
1.66 |
5.38×10−6 |
3.55×10−6 |
According to the physical adsorption isotherm classification proposed by IUPAC [31], the hypothermic N2 adsorption isotherms of the Br-doped V/TiO2 showed hysteresis loops, and capillary agglomeration occurred, which belonged to type IV isotherms. This isotherm was produced by mesopores, which consistent with the measured average pore diameter (between 7 and 17 nm). From the hysteresis loops, the change of the adsorption amount was not very steep but rather slow, indicating that the pore distribution range was relatively wide, and it should be a mixture of the cylindrical hole with open ends and the slit hole with parallel plate structure. When the relative pressure (P/P0) was low, the gas diffuses in the pores with a pore diameter of 1.5~100 nm, which is generally Knudsen diffusion.
In this study, N2, O2 and Hg were diffused in the Br-doped V/TiO2 catalyst by Knudsen diffusion. The resistance comes from the collision of molecules with the pore walls, and the diffusion coefficient Dk mainly depends on the temperature T and the pore radius r.
Figure 2: N2 adsorption-desorption curves of catalysts.
Crystal Structure Analysis
The XRD data was obtained in (Figure 3). The diffraction peak of the anatase phase TiO2 (JCPDS 21-1272) in the bromine-doped catalyst were narrower and stronger than that of the undoped Br sample (VTiBr0). Br may promote the formation of the crystal. In addition, compared with VTiBr 0, the diffraction angle of the anatase phase TiO2 on the Br-doped V/TiO2 becomes smaller, and the interplanar spacing of the crystal increases. According to the Bragg diffraction formula, this indicates that a solid solution is formed on the Br-doped catalyst.
Figure 3: XRD analysis results of catalysts.
Element valence analysis
Figure 4 showed the EPR spectrum of the Br-doped V/TiO2 products at room temperature, which manifested an amount of V4+ ions grew on its surface and bulk phase. Although the loading of vanadia was low (see Table 1), peaks belonging to V4+ ions can be clearly detected. By integrating the peaks of V4+ ions, the peak area reduced in the following order: VTiBr 1.2> VTiBr 1.6 > VTiBr 0.8 > VTiBr 2 > VTiBr 0.4 > VTiBr 0. With the increasing of the Br-doping, the generated V4+ ions VTiBr 1.2 were the most among the Br-doped V/TiO2 samples.
Figure 4: EPR spectra of different Br-doped content catalysts.
Figure 5 showed the XPS spectra of the samples. As can be seen from Figure 5(d), after the bromine was doped, the peaks shifted toward the low binding energy direction. Figure 5 showed that the Ti 2p3/2, V 2p3/2 and O 1s peaks of VTiBr 1.2 were shifted by about 0.2 eV, 0.5 eV and 0.3 eV, respectively, in the direction of low binding energy compared to the sample VTiBr 0. This meant that the electrons was lost on Ti and V, and the electrons obtained by O, so, the valence states of Ti and V were not completely +4 and +5, respectively. In addition, since the amount of doping of bromine was small, no significant Br information was obtained in Figure 5(d).
Figure 5: XPS spectra of the catalysts.
PL spectral analysis
In order to further discuss the effect of bromine on the structure of the catalyst, the photoluminescence properties of the samples were studied. In general, the emission peak of anatase TiO2 is about 380 nm. It can be seen from (Figure 6) that after the introduction of bromine, the emission peak at about 380 nm moved toward the long wavelength direction, which showed the molecular rigidity increased. The emission peak at about 376 nm moved to the short wavelength direction, and the emission peak at around 376 nm was enhanced after the addition of bromine. Typically, electron withdrawing groups weaken or even quench fluorescence. Oxygen has a higher electron absorption capacity than bromine, and fluorescence should be enhanced after bromine doping [32]. Therefore, the emission peak at around 376 nm should be related to the electron-withdrawing group oxygen and bromine.
Figure 6: PL spectra of the catalysts.
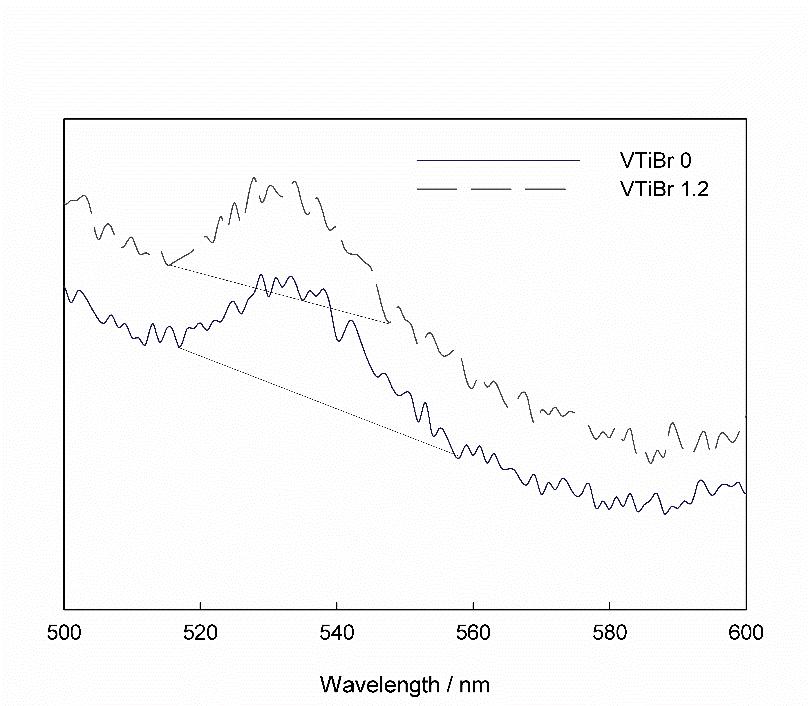
The peak at 530 nm in Figure 7 indicated the presence of a color center with one electron [33-37], which was still present but reduced after bromine doping. Since oxygen adsorption to the color center can reduce the luminescence properties of the sample [38], the luminescence peak of the bromine-doped sample at 530 nm was weaker than that of the undoped sample, indicating the difference in oxygen adsorption capacity between the two samples, that is, the bromine-doped sample can adsorb more oxygen than the undoped sample.
Figure 7: PL spectra around 530 nm of the catalysts.
From the characterization results of EPR, XPS and PL, it can be seen that the samples in this study contained mixed valences of Ti and V, and bromine doping could increase the amount of V4+ and Ti3+, that is, the reduction characteristics or redox characteristics of the catalysts after bromine doping were improved [39-42].
Elemental mercury oxidation performance
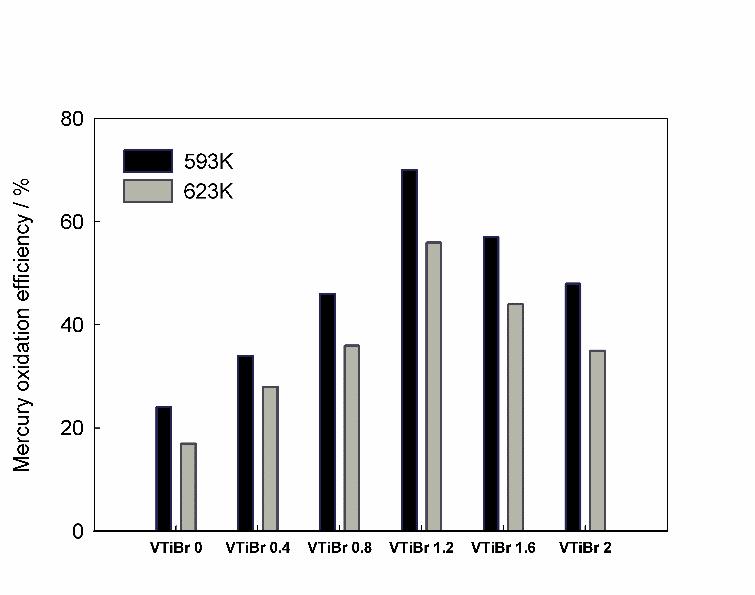
In order to evaluate the effect of bromine doping on the mercury oxidation performance, the elemental mercury oxidation activity of the catalyst was tested with the simulated flue gas. The results (Table 1 and Figure 8) showed that bromine could significantly improve the mercury oxidation performance. As the bromine doping amount increased, the activity first increased and then decreased, and the VTiBr 1.2 sample has the highest mercury oxidation efficiency. In addition, the mercury oxidation performance decreased with temperature increasing, possibly the high temperature may Inhibit the adsorption of mercury on the samples.
In the mercury oxidation performance test, there was no hydrogen halide involved such as HCl, thus, it is no longer necessary to spray hydrogen halide into the flue gas under the actual application condition, which avoided the corrosion of hydrogen halides on the equipment, thus also reduced the operating costs. The test results also validated our catalyst design ideas.
Figure 8: Activity test results of elemental mercury oxidation.
Testing conditions: the simulated flue gas flow rate was 150 ml/min, and its composition was 3×10-5 μg/ml Hg + 5% O2, and the rest was N2. Catalyst usage 0.3 g.
CONCLUSIONS
In order to reduce the mercury control cost of coal-fired flue gas, it is an effective and feasible way to control mercury by SCR denitrification synergistic oxidation. By analyzing the importance of non-metallic bromine in the existing mature mercury removal technology, this paper studied the effect of non-metallic bromine doping on the structure and properties of vanadia/titania catalyst from a non-metallic perspective, exploring new ideas for elemental mercury oxidation by SCR catalysts. The results showed that after bromine doping, the samples in this study contained mixed valences of Ti and V, and the bromine doping could increase the amount of V4+ and Ti3+, that is, the reduction characteristics or redox characteristics of the catalysts after bromine doping were improved. According to the basic theory of gas-solid phase catalytic reaction, the increase of the redox capacity of the catalyst enhances the catalytic activity of the catalyst, which was verified in the activity test. As the bromine doping amount increased, the activity first increased and then decreased, and the VTiBr 1.2 sample has the highest mercury oxidation performance.
Acknowledgments
The authors want to thank the financial support from the Scientific and Technological Research Program of the Chongqing Municipal Education Commission (KJ1711266/ KJ1711286/ KJ1711290) and the Research Program of Chongqing University of Arts and Sciences (No: R2016CH07/ No: 2017RCH07). We gratefully acknowledge the many important contributions from the researchers of all the reports cited in this paper.
REFERENCES
- Heidel B, Rogge T, Scheffknecht G (2016) Controlled desorption of mercury in wet FGD waste water treatment. Appl Energy 162: 1211-1217.
- Lee C, Serre S, Zhao Y, Lee SJ, Hastings TW (2006) Study of the effect of chlorine addition on mercury oxidation by SCR catalyst under simulated subbituminuous coal flue gas. In Proceedings, EPA-DOE-EPRI-A&WMA Power Plant Air Pollutant Control "Mega" Symposium, Baltimore, MD, USA [Internet].
- Schwammlea T, Bertschea F, Hartungb A, Brandensteinc J, Heidela B, Scheffknechta G (2013) Influence of geometrical parameters of honeycomb commercial SCR-DeNOx-catalysts on DeNOx-activity, mercury oxidation and SO2/SO3-conversion. Chem Eng J 222: 274-281.
- Wang Z, Liu J, Zhang B, Yang Y, Zhang Z, Miao S (2016) Mechanism of heterogeneous mercury oxidation by HBr over V2O5/TiO2 catalyst. Environ Sci Technol 50 (10): 5398-5404.
- Zhang B, Liu J, Yang Y, Chang M (2015) Oxidation mechanism of elemental mercury by HCl over MnO2 catalyst: Insights from first principles. Chem Eng J 280: 354-362.
- Zhao B, Liu X, Zhou Z, Shao H, Xu M (2016) Catalytic oxidation of elemental mercury by Mn–Mo/CNT at low temperature. Chem Eng J 284: 1233-1241.
- Zhao S, Li Z, Qu Z, Yan N, Huang W, Chen W, et al. (2015) Co-benefit of Ag and Mo for the catalytic oxidation of elemental mercury. Fuel 158: 891-897.
- Zhou Z, Liu X, Zhao B, Shao H, Xu Y, Xu (2016). M Elemental mercury oxidation over manganese-based perovskite-type catalyst at low temperature. Chem Eng J 288: 701-710.
- Zhao L, Li C, Zhang J, Zhang X, Zhan F, Ma J, et al. (2015) Promotional effect of CeO2 modified support on V2O5–WO3/TiO2 catalyst for elemental mercury oxidation in simulated coal-fired flue gas. Fuel 153: 361-369.
- Zhang A, Zheng W, Song J, Hu S, Liu Z, Xiang J (2014) Cobalt manganese oxides modified titania catalysts for oxidation of elemental mercury at low flue gas temperature. Chem Eng J 236: 29-38.
- Ling L, Zhao Z, Zhao S, Wang Q, Wang B, Zhang R, et al. (2017) Effects of metals doping on the removal of Hg and H2S over ceria. Appl Surf Sci 403: 500-508.
- Yang J, Yang Q, Sun J, Liu Q, Zhao D, Gao W, et al. (2015) Effects of mercury oxidation on V2O5–WO3/TiO2 catalyst properties in NH3-SCR process. Catal Commun 59: 78-82.
- Zhao L, Li C, Zhang X, Zeng G, Zhang J, Xie Y (2015) A review on oxidation of elemental mercury from coal-fired flue gas with selective catalytic reduction catalysts. Catal Sci Technol 5: 3459-3472.
- Yang Y, Liu J, Zhang B, Zhao Y, Chen X, Shen F (2017) Experimental and theoretical studies of mercury oxidation over CeO2 − WO3/TiO2 catalysts in coal-fired flue gas. Chem Eng J 317: 758-765.
- Jampaiah D, Tur KM, Venkataswamy P, Ippolito SJ, Sabri YM, Tardio J, et al. (2015) Catalytic oxidation and adsorption of elemental mercury over nanostructured CeO2–MnOx catalyst. RSC Advances.- 5(38): 30331-30341.
- Chen W, Ma Y, Yan N, Qu Z, Yang S, Xie J, et al. (2014) The co-benefit of elemental mercury oxidation and slip ammonia abatement with SCR-Plus catalysts. Fuel 133: 263-269.
- Songjian Zhao, Wanmiao Chen, Wenjun Huang, Jiangkun Xie, Zan Qu, Naiqiang Yan (2018) Elemental mercury catalytic oxidation removal and SeO2 poisoning investigation over RuO2 modified Ce-Zr complex. Appl Catal A Gen 564: 64-71.
- Dranga B, Koeser H (2015) Increased co-oxidation activity for mercury under hot and cold site coal power plant conditions – Preparation and evaluation of Au/TiO2-coated SCR-DeNOx catalysts. Appl Catal B 166-167: 302-312.
- Liu Z, Li X, Lee J-Y, Bolin T B (2015) Oxidation of elemental mercury vapor over γ-Al2O3 supported CuCl2 catalyst for mercury emissions control. Chem Eng J 275: 1-7.
- Yongpeng Ma, Bailong Mu, Xiaojing Zhang, Dongli Yuan, Chuang Ma, Haomiao Xu, et al. (2019) Graphene enhanced Mn-Ce binary metal oxides for catalytic oxidation and adsorption of elemental mercury from coal-fired flue gas, Chem Eng J e 358: 1499-1506.
- Yang Y, Liu J, Wang Z, Liu F (2017) Heterogeneous reaction kinetics of mercury oxidation by HCl over Fe2O3 surface. Fuel Process Technol 159: 266-271.
- Huang W, Xu H, Qu Z, Zhao S, Chen W, Yan N (2016) Significance of Fe2O3 modified SCR catalyst for gas-phase elemental mercury oxidation in coal-fired flue gas. Fuel Process Technol 149: 23-28.
- Li H, Wang S, Wang X, Hu J (2017) Activity of CuCl2-modified cobalt catalyst supported on Ti-Ce composite for simultaneous catalytic oxidation of Hg0 and NO in a simulated pre-sco process. Chem Eng J 316: 1103-1113.
- Yang J, Zhao Y, Chang L, Zhang J, Zheng C (2015) Mercury adsorption and oxidation over cobalt oxide loaded magnetospheres catalyst from fly ash in oxyfuel combustion flue gas. Environ Sci Technol 49 (13): 8210-8218.
- Xi Liu, Shaojian Jiang, Hailong Li, Jianping Yang, Zequn Yang, Jiexia Zhao, et al. (2019) Elemental mercury oxidation over manganese oxide octahedral molecular sieve catalyst at low flue gas temperature. Chem Eng J 356: 142-150.
- Zhen Zhang, Jiang Wu, Bin Li, Huibin Xu, Dongjing Liu (2019) Removal of elemental mercury from simulated flue gas by ZSM-5 modified with Mn-Fe mixed oxides. Chem Eng J.375: 121946.
- Stollea R, Koesera Heinz, Heinz Gutberlet (2014) Oxidation and reduction of mercury by SCR DeNOx catalysts under flue gas conditions in coal fired power plants. Appl Catal B 144: 486-497.
- Lingkui Zhao, Caiting Li, Xunan Zhang, Guangming Zeng, Jie Zhang, Yin'e Xie (2015) A review on oxidation of elemental mercury from coal-fired flue gas with selective catalytic reduction catalysts, Catal Sci Technol 5(7): 3459-3472.
- Valdés-Solís T, Marbán G, Fuertes AB (2003) Low- temperature SCR of NOX with NH3 over carbon-ceramic supported catalysts. Appl Catal B 46(2): 261-271.
- Chen K, Xie S, Iglesia E, Bell AT (2000) Structure and properties of zirconia-supported molybdenum oxide catalysts for oxidative dehydrogenation of Propane. J Catal 189(2): 421-430.
- Matthias Thommes, Katsumi Kaneko, Alexander V. Neimark, James P. Olivier, Francisco Rodriguez-Reinoso, Jean Rouquerol, et al. (2015). Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report), Pure appl Chem 87(9-10): 1051-1069.
- Notestein, JM, Iglesia E, Katz A (2007) Photoluminescence and Charge-Transfer Complexes of Calixarenes Grafted on TiO2 Nanoparticles, Chem Mater 19(20): 4998-5005.
- Li D, Haneda H, Hishita S, Ohashi N, Labhsetwar NK (2005) Fluorine-doped TiO2 powders prepared by spray pyrolysis and their improved photocatalytic activity for decomposition of gas-phase acetaldehyde J Fluor Chem. 126(1): 69-77.
- Li D, Haneda H, Labhsetwar NK, Hishita S, Ohashi N (2005) Visible-light-driven photocatalysis on fluorine-doped TiO2 powders by the creation of surface oxygen vacancies. Chem Phys Lett 401(4-6): 579-584.
- Li D, Ohashi N, Hishita S, Kolodiazhnyi T, Haneda H (2005) Origin of visible-light-driven photocatalysis: A comparative study on N/F-doped and N-F-codoped TiO2 powders by means of experimental characterizations and theoretical calculations. J Solid State Chem 178(11): 3293-3302.
- Lei Y, Zhang LD, Meng GW, Li GH, Zhang XY, Liang CH, et al. (2001) Preparation and photoluminescence of highly ordered TiO2 nanowire arrays. Appl Phys Lett 78(8): 1125-1127.
- Rui Zhang, Qin Zhong, Wei Zhao, Lemeng Yu, Hongxia Qu (2014) Promotional effect of fluorine on the selective catalytic reduction of NO with NH3 over CeO2-TiO2 catalyst at low temperature. Appl Surf Sci 289: 237-244.
- Anpo M, Che M, Fubini B, Garrone E, Giamello E, Paganini MC (1999) Generation of superoxide ions at oxide surfaces. Top Catal 8(3-4): 189-198.
- Narayana K V, Raju BD, Masthan SK, Rao VV, Rao PK, Subrahmanian R, et al. (2004) ESR spectroscopic characterization of V2O5/AlF3 ammoxidation catalysts. Catal Commun 5(8): 457-462.
- Scheurell K, Kemnitz E (2005) Amorphous aluminium fluoride as new matrix for vanadium-containing catalysts. J Mater Chem 15(45): 4845-4853.
- Scheurell K, Scholz G, Kemnitz E (2007) Structural study of VOx doped aluminium fluoride and aluminium oxide catalysts. J Solid State Chem 180(2): 749-758.
- Scheurell K, Scholz G, Pawlik A, Kemnitz E (2008) VOx doped Al2O3 and AlF3 -a comparison of bulk, surface and catalytic properties. Solid State Sci 10(7): 873-883.
Copyright: Li Y. © 2019. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation: Li Y (2019). The Characterization and Mercury Oxidation Performance of Bromine-Doped Vanadia/Titania Oxide Catalyst. Material Sci 1(1): 2.
 Abstract
Abstract  PDF
PDF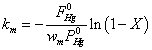
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)
.jpg)