Current Issue
Stone Matrix Asphalt (SMA) and pH Instability Shown on Hamburg Wheel Tracker Test Failures of SMA - Case Study
James Allen Cox*
Associate Professor, Department of Construction Technologies, Utah Valley University, USA
*Corresponding author: James Allen Cox, PhD, PLS, Associate Professor, Department of Construction Technologies, Utah Valley University, 800 West University Avenue, Orem, Utah 84058, USA, Phone: 8018851741, E-mail: [email protected]
Received Date: May 20, 2025
Publication Date: June 30, 2025
Citation: Cox JA. (2025). Stone Matrix Asphalt (SMA) and pH Instability Shown on Hamburg Wheel Tracker Test Failures of SMA - Case Study. Material Science. 7(1):40.
Copyright: Cox JA. © (2025).
ABSTRACT
Stone Matrix Asphalt (SMA) is a combination of igneous, metamorphic and sedimentary aggregate materials from various sources blended with asphalt binders and mineral fillers. These components are sometimes modified by various additives to enhance the bonding of the binders and the aggregates. Concern over failed Hamburg Wheel Tracker tests of strength and durability in the Stone Matrix Asphalt mixtures prompted research into what failure mechanism is being experienced on these projects. One of the six stripping mechanisms in these failures is the pH at the interface of the binder and the aggregate. This research will assess moisture damage or stripping associated with pH instability of the individual components of a loose blended SMA mix with other types of tests associated with the corresponding material sources. The main hypothesis is that pH of the mixed components of binder and aggregate may be an indicator of potential early stripping of the binder from the aggregates. On a production level, those binder-aggregates mixes high in pH could indicate a potential for failure by any number of adhesion theory failure mechanisms that weakens the bond between the asphalt binder and the aggregate causing the SMA to fail in the field. One failure mechanism found in concrete (alkali-silica reactivity of the aggregate) could be a contributor in SMA mixes and will be appraised in this case study. The study of the failed SMA mixes may support this possibility. To accomplish this case study, the following will be addressed:
• A review of the two project mix designs and test failures
• Tests of pH were conducted on the aggregate source, of prepared loose mix and additives to be used in the SMA mixes.
• An assessment of only a limited number of Hamburg Wheel Tracker test data of the SMA placed on the project as well as the gradations, densities and oil content to determine which components are contributing to the failures.
• The results of this research will be compared with my dissertation research paper on the pH of aggregate sources in Utah.
Such research would be a resource to any department of transportation and any contractor in selecting appropriate binder and aggregate combinations to ensure the SMA mixes will result in a longer lasting, quality pavement.
Keywords: Aggregates, Asphalt, pH Instability or Reactivity, Hamburg Wheel Tracker Test Results, Moisture Damage or Stripping, Adhesion or Bond
INTRODUCTION
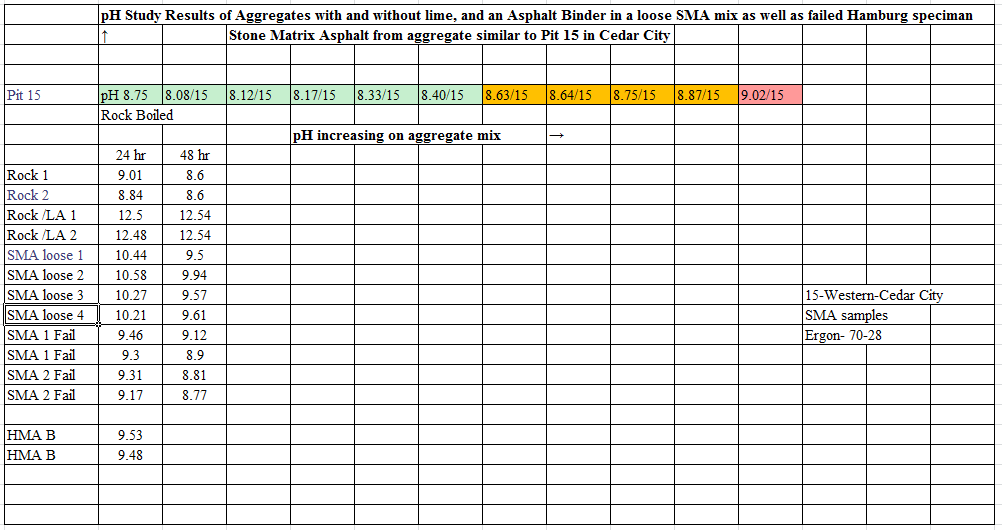
The summer of 2012 the Utah Department of Transportation (UDOT) used Stone Matrix Asphalt (SMA) to pave projects in Region Four, Cedar City area. The contractor had produced the SMA aggregate from the source across a road in the same aggregate layer as source 15 used in chapter three of my dissertation published at the University of Utah in May 2016. It shows the chemical composition of the aggregate as 31% SiO 2, 56% CaCO3 and 37% CaO. This indicates a carbonate water reaction which could result in stripping of the asphalt from the aggregate. The UDOT lab in Richfield, in testing the material, found that after it had been placed on the roadway the Hamburg Wheel Tracker tests were failing. The purpose of this case study is to relate pH of the aggregate source and to evaluate the failed Hamburg Wheel Tracker field tests. Samples of the loose unmixed aggregate, the loose aggregate with mineral filler mixed into it and the SMA combined mix were obtained. The test results of two failed Hamburg Wheel Tracker specimens were provided. These samples were all tested in the same manner as the samples in chapter three of my dissertation research paper [1]. See Appendix A, Table 16 for comparison results.
The significance and importance of this case study is that it verifies previous testing of this aggregate source at this location.
METHODOLOGY
The first objective was to assess the relationships of the components used in the SMA mixes to determine their individual contribution to the final pH within the SMA mix. The chemistry of the aggregate can, when combined with lime, create a pH environment at the asphalt binder –aggregate interface conducive to the bond being compromised. The overall biggest factor affecting final pH is the addition of hydrated lime to the SMA mix, which is to be expected. Those mixes to which hydrated lime had been added exhibited a higher mean value of pH but also test with a much greater variability. Aggregate components such as SiO2, CaCO3 and CaO influence final pH and add or subtract from the final analysis. Knowledge of aggregate chemistry and its potential to react with lime would improve the overall success of the bond and a longer lasting pavement. ASR/ACR should be investigated in suspect pits.
The second objective was to determine if the final pH of the SMA mix is a significant predictor of chemical reaction, which may lead to stripping damage in the asphalt binder/aggregate mix. The issue of stripping damage has not been evaluated in this work since the tests failed to pass the field testing done on the SMA mix.
Hydrated lime has a direct effect on the final pH of an SMA mixture of materials and when combined with certain percentages and types of silicas and carbonates can combine to increase or decrease the pH level. The use of hydrated lime in most mixes (85-90%) helps the bonding and improves the adhesion. In 10-15% of the mixes, the chemistry of the aggregate factors into the equation and combined with the lime creates chemical reactions in the mix, as indicated by exchange of H+ ions and OH-, causing an unstable interface between the asphalt binder and the aggregate. The higher pH values do predict detrimental chemical reactivity in the asphalt binder/aggregate mix. See test results for amount of lime added to the mix and for mix preparation.
RESULTS
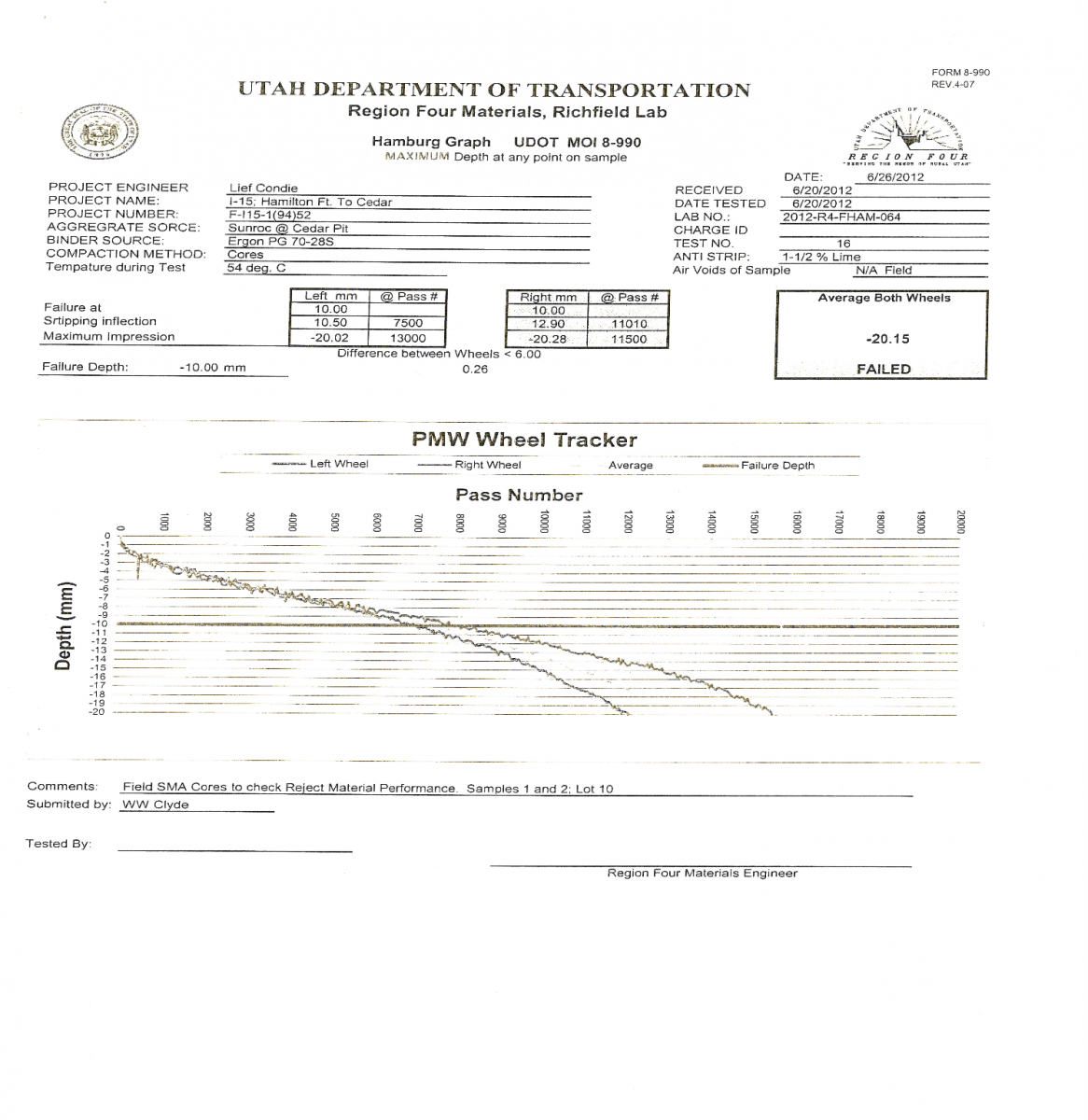
The results are shown in Table 1. The results of the failed Hamburg tests from lot 10 on the project are shown in Figures 1 and 2. (The aggregates for the two failed samples came from Cedar City pit #15 and have a pH as shown below).
Table 1. Stone Matrix Asphalt pH study Results
Figure 1. Lot 10 samples 1and 2 failures.
Figure 2. Lot 10 samples 3 and 4 failure.
DISCUSSION OF RESULTS
The Hamburg Wheeler Tracker tests shown above are used to accept the SMA product according to ASTM test standards. This test requires the sample to be tested for 20,000 passes of two steel wheels each 156 lbs. under water at a 72-degree temperature. If during the test the specimen ruts more than 10 mm fails, the test requires the sample to not rut more than 10 mm at 20,000 passes of the steel wheels to pass acceptance of the product [2]. At 20,000 passes each of the above samples had rutted more than 20 mm. If the specimen fails it may correlate with the high pH value of the aggregate which cause stripping of the asphalt from the aggregate causing the rutting.
CONCLUSION
The first conclusion is that the pH of individual components of aggregates, asphalt binder and additives point to and isolate suspect materials which chemically react and affect the Hamburg testing results.
Components such as hydrated lime, which is an additive and the chemistry of the aggregate (SiO2, CaCO3 and CaO) had the most significant effect on final pH value. The chemical composition of the aggregate in the source pit will have a positive or negative effect on the pH value depending on the percentages of each mineral.
The second conclusion is that pH levels of mixed SMA materials (asphalt binders, additives and aggregates) have been significant in predicting chemical reaction, which is suspected to affect the durability of the asphalt binder/aggregate mix.
The chemical composition of this aggregate source has a significant effect on the failure of the Hamburg tests because of stripping due to the pH at the interface of the aggregate and the water layer between the binder and aggregate [1].
Future research is needed on the drying of the aggregate to remove all moisture prior to adding the asphalt to stop chemical reaction of the aggregate to produce a more stable mixture. Aggregate source suitability research testing for aggregate chemistry would also be recommended [3-6].
ACKNOWLEDGEMENTS
None.
CONFLICT OF INTEREST
The author declares that there is no conflict of interest.
REFERENCES
- Cox JA. (2016). Significance of pH Variance in Predicting Chemical Reaction in Hot Mix Asphalt. Journal of Materials in Civil Engineering. 28(3). DOI: 10.1061/(ASCE)MT.1943-5533.0001391.
- Cox JA. (2013). On the Variability of Results from the Hamburg Wheel Tracker Device. Associated Schools of Construction Journal. pp. 263-274.
- Barbour AB. (1974). A study of asphalt-aggregate interactions using inverse-chromatography. Journal of Applied Chemistry and Biotechnology. 24(11):645-654.
- Brannan CJ. (1991). Adsorption behavior of asphalt models and asphalts on siliceous and calcareous aggregates. Transportation Research Board. 1393:10-21.
- Izzo RA. (1997). Use of the Hamburg Wheel-Tracking Device for Evaluating Moisture Susceptibility of Hot Mix Asphalt- Paper No. 99-0955. Austin, Texas: Transportation Research Record 1681.
- Solaimanian MA. (2000b). Relationship Between Aggregate Properties and Hamburg Wheel tracking Results. Austin: Center for Transportation Research- University of Texas- Research Report 4977-1F.
 Abstract
Abstract  PDF
PDF